Snow, hail, bacteria and nucleating ice: bioprecipitation part II
Ice crystals make more ice crystals in clouds. Bacteria make more bacteria when wet.
This is an article that can be read on its own, or as a continuation of previous article Bioprecipitation part I.
With the dawn of the new millennium, three key experimental measurements helped advance the bacteria-seeding-cloud paradigm: the first related to snow, the second to finding the ratio of bacteria to other ice-nucleating particles, and the third to hail.
The first key experimental measurement took place in 2005 when David Sands decided to revisit the question of whether bacteria played a significant role in weather, following his work on the topic in the 1970s and early ’80s. To do so, he needed collaborators. He recruited Brent Christner, a post-doc also working at his university, Montana State. Christner had a penchant for studying microbes that lived in extreme conditions, having researched them under both very hot and very cold conditions, such as those found in Antarctica. Sands presented him with another extreme condition: the atmosphere, where there was nothing for the bacteria to bind to, where the sun’s radiation beat down relentlessly, and where winds and storms blew them around. Also joining the research team was Cindy Morris, a plant pathologist who specialized in the Pseudomonas syringae bacteria and who had a fondness for turning environmentally related thoughts into song.
They studied snow. Snow had the advantage over rain in clarifying bacteria’s ability to nucleate water vapor into ice because rain can form in two ways: one where the water vapor nucleates into liquid water droplets, and another where it nucleates into ice. Snow, on the other hand, is formed only in one way—when water vapor nucleates into ice.
They gathered snow from Montana, Yukon, France, and Antarctica, and examined it to see if it contained biological material. Indeed, it did. All the snow contained biological nucleators capable of nucleating water vapor into snow at temperatures between -7°C and -4°C, temperatures higher than those at which inorganic particles could nucleate snow. Montana and France had the most biological nucleators in the snow, followed by Yukon and then Antarctica. Their results showed that snow might be seeded by biological material worldwide. [Christner 2008]
However, there was also a puzzle here if the bacteria-seeding-cloud paradigm was to hold. The Montana and France samples had about 100 nucleators per liter of water after the snow melted, which, at first glance, doesn’t seem like enough for the bacteria to be seeding a significant amount of the rain, given how many raindrops would be in a liter of water if each nucleator seeded only one raindrop.
However, insight into this puzzle came from ongoing research on cloud ice nucleators in general, a topic that includes both inorganic and organic ice nuclei. The insights came from increasingly sophisticated devices that could peer more closely at what was happening in clouds, and from better lab simulations of cloud formation. They showed that the impact of one ice nucleus did not end at just nucleating one ice crystal; instead, it could trigger an avalanche of reactions leading to a plethora of ice crystals. The initially seeded ice crystal, once formed, would cause supercooled water droplets that came into contact with it to freeze as well. At temperatures between -8°C and -3°C, the supercooled water could turn into ice splinters, which would then break apart into many pieces, generating many more ice crystals (this sequence of events was named the Hallet-Mossop process). Updrafts could then blow some of these smaller ice crystals upward, where they, in turn, attract more supercooled water to turn into ice, which would then break apart into many more new parts. Through this process, a few bacteria could seed ice crystals numbering orders of magnitude more.
The second experimental measurement to advance the bacteria-seeding-cloud paradigm also benefited from better measurement equipment. Kim Prather, an atmospheric chemist, had spent 15 years creating and fine-tuning a machine that could suck up microscopic particles and then efficiently determine their chemical composition. She teamed up with Paul DeMott, another atmospheric chemist. DeMott, while an undergrad in the 1970s, had been inspired to work on ice nucleation by his connection with the scientist Bernard Vonnegut, who studied atmospheric ice. Bernard’s brother, Kurt Vonnegut, inspired by this atmospheric ice work, wrote about Ice-9, a substance that froze water at room temperature, in his 1963 novel Cat’s Cradle.
Using the machine that Prather had developed and flying at heights of up to 25,000 feet over Colorado, Wyoming, and Montana, Prather and DeMott gathered aerosols (another name for particles aloft in the air) from the clouds and then sorted them to determine their composition. Of the ice nucleation particles they found, 50% were composed of dust from Africa and Asia, and about 33% from biological matter [Prather 2009]. It seemed bacteria thus made up a decent proportion of ice-nucleating particles and should therefore have a significant impact on cloud formation
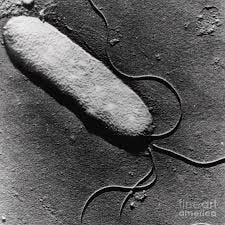
[Pseudomonas Syringae]
Other experimental measurements also supported this prevalence of biological nucleators. Anthony Prenni found that biological particles made up a significant proportion of ice-nucleating particles over the Amazon during the season [Prenni 2009].
However, some simulation results seemed to find a smaller proportion. Corrina Hoose’s simulations [2010] showed that bacteria comprised only a small fraction of one percent of the ice nucleators. Dominick Spracklen [2014], an environmental professor at the University of Leeds, also discovered a similar small fraction, but his simulations indicated that bacteria and fungal spores did play a significant role in seeding cloud ice crystals at lower altitudes of around 5 km (400 to 600 hPa pressure ranges in the atmosphere would be a more accurate way of describing the altitude) because they could nucleate in the warmer temperatures of lower altitudes where other ice nucleating particles could not.
I wonder if the difference in ice nucleator proportions between experiments and simulations is because the simulations assumed a much more uniform distribution of bacteria, whereas in reality, the distribution is not uniform, with bacteria being much more likely to be present in clouds. Convection patterns and cloud formation pathways might lead to more bacteria finding their way into clouds. When confronted with a difference between measurement and simulation in these kind of situations, measurements are probably the way to go.
The third experimental measurement that hailed the advance of the bacteria-seeding-cloud paradigm came from the study of hail.
Alexander Michaud, a graduate student at Montana State University, where David Sands also worked, was primarily studying microbes in Antarctica. One day, however, hail the size of golf balls from Rocky Mountain storms hit the campus, smashing into houses and buildings and causing millions of dollars in damages. This event prompted Michaud to investigate what seeded the hail. He collected the hailstones and cut them into very thin slices. The hail was made up of many layers, like an onion, and in the core, he found, interestingly, that most of the bacteria in the hail resided there. This was strong evidence that the bacteria were causing the nucleation; otherwise, there would have been similar concentrations of bacteria in every layer.
Hail, like snow, is a form of solid matter, and the solid state is helpful for figuring out the past. Solid matter has the ability to trap history within its structure. The liquid state of water, due to its fluidity, loses memory of its past. We know much about organisms that lived millions of years ago because they were encased in solid ice.
To figure out what happened at the moment of nucleation, Michaud consulted Montana State professor Berry Lyons. The solidity of the hailstone helped preserve information about that moment. When they melted the hailstone, a vapor was released, which they analyzed with what’s called a Picarro analyzer. From the isotopic composition of the vapor, they could tell that the hailstone froze at temperatures higher than -14°C (6.8°F).
The diagram below shows general information from another experiment [Murray 2012] about the number of ice-nucleating sites of each substance. At temperatures higher than -14°C, only bacteria and pollen can nucleate ice. Inorganic substances only nucleate ice at lower temperatures.
The fact that the hailstones froze above -14°C indicated that biological material likely seeded them.
In addition to these three key experiments, other clues emerged that supported the bacteria-seeding-cloud paradigm. Pseudomonas syringae traveled shorter distances in the air than bacteria in general, suggesting they were seeding their own rain that then brought them out of the sky. Furthermore, the rate of ice formation in clouds mimicked the rate of ice formation by Pseudomonas syringae in labs, suggesting the causal factor was the same.
...
Ice-nucleating bacteria introduced new insights into our understanding of the small water cycle.
In the 1950s, Keith Bigg, an atmospheric physicist, noticed something very odd about the behavior of ice nucleators after rain events in Australia. The numbers would keep increasing for 21 days afterward, but Bigg could think of no reason why the nuclei would increase in number. He continuously racked his brain over the problem.
When he discussed the issue with Cindy Morris in 2011, she thought the post-rain ice nuclei numbers behaved like phenomena she had seen in population ecology. This led her to realize that the ice-nucleating particles were increasing in numbers because the bacteria were self-replicating. Upon hearing this, Bigg felt a sense of relief; after all these years, he wasn’t going crazy by being so intrigued by and so pursuant of this anomalous behavior.
Pseudomonas syringae grow better in the presence of water. With rain coming down, they would increase their numbers for about 21 days. Mists created from the rain splash would then carry the bacteria aloft.
This bacterial self-replication behavior explained why, in 1970, when Russ Schnell gathered plants and leaves and washed them with distilled water, the distilled water did not initially nucleate at higher temperatures. However, when he accidentally left the leaf litter for ten days, the distilled water it was in turned turgid and began to nucleate at higher temperatures. The bacteria had been given time to multiply into more ice nucleators.
More Pseudomonas syringae bacteria in the air days after a rain means they could seed more rain, provided there is enough humidity in the air and the air is not already saturated with ice nucleators. ( See Cindy Morris’s thoughts on this matter in her biological nucleator blog)
...
David Sands surmises that one of the causes of the world’s increased drought frequency is certain human activities that decrease Pseudomonas syringae numbers, such as the overuse of pesticides and overgrazing of land. Increasing Pseudomonas syringae in agriculture is a delicate matter because the bacteria can also cause crop damage. Sands suggests that selecting the right strains of soybeans and wheat, combined with Pseudomonas syringae cultivation, could help increase rainfall.
Increasing rainfall depends on both increasing water vapor and having enough rain nucleators. Cindy Morris suggests planting strips of vegetation at the base of mountains so that they release more biological nucleators, which are then carried by air currents up the mountain. The biological nucleators can then help seed the clouds that often form at the tops of mountains and encourage them to rain.
Pseudomonas syringae’s preference for humid conditions also suggests that we should try to keep more water in the soil through the use of mulch and by not clearing forest floors. In addition the bacteria need plant matter to self-replicate, so leaving fallen leaves on the ground can increase their habitat. The ways of understanding how to use bacteria to increase the small water cycle is still a nascent science.
………………………
Part III is here
………………………
Are you interested in how its possible to increase the rain in your province, state, bioregion? Increasing the rain can help with the hydration of the land, increasing the water supply, aiding agriculture and lessening wildfires. We (Ali Bin Shahid and I ) are offering a new service where we do rough calculations for the amount of ecorestoration that has to be done in order to increase the rain x amount. A water plan is presented for your bioregion which gives numbers on how much the eco-restoration of forests, wetlands, grasslands will increase the small water cycle. We point out different opportunities for water cycle restoration. The service is sliding scale $100-$500. This is an initial trial of this service. If interested you can contact me by replying to this email.
References
Bigg, E. Keith, Samuel Soubeyrand, and Cindy E. Morris. "Persistent after-effects of heavy rain on concentrations of ice nuclei and rainfall suggest a biological cause." Atmospheric Chemistry and Physics 15, no. 5 (2015): 2313-2326.
Burrows S. M , Elbert W , Lawrence M. G , Poschl U . Bacteria in the global atmosphere – Part 1: Review and synthesis of literature data for different ecosystems . Atmos. Chem. Phys . 2009a ; 9 : 9263 – 9280. {Contains map of the bioaerosols around the globe}
Christner, Brent C., Cindy E. Morris, Christine M. Foreman, Rongman Cai, and David C. Sands. "Ubiquity of biological ice nucleators in snowfall." Science 319, no. 5867 (2008): 1214-1214.
Hoose, C., J. E. Kristjánsson, and S. M. Burrows. "How important is biological ice nucleation in clouds on a global scale?." Environmental Research Letters 5, no. 2 (2010): 024009.
Huang, Shu, Wei Hu, Jie Chen, Zhijun Wu, Daizhou Zhang, and Pingqing Fu. "Overview of biological ice nucleating particles in the atmosphere." Environment International 146 (2021): 106197.
Joung, Y., Ge, Z. & Buie, C. Bioaerosol generation by raindrops on soil. Nat Commun 8, 14668 (2017). https://doi.org/10.1038/ncomms14668
Joyce, R. E., Lavender, H., Farrar, J., Werth, J. T., Weber, C. F., D’Andrilli, J., Vaitilingom, M., and Christner, B. C. 2019. Biological ice-nucleating particles deposited year-round in subtropical precipitation. Applied and Environmental Microbiology, 85:e01567-19.
Morris C, Monteil C, Berge O “The Life History of Pseudomonas syringae: Linking Agriculture to Earth System Processes” Annual Review of Phytopathology Vol. 51:85-104 (August 2013) https://doi.org/10.1146/annurev-phyto-082712-102402
Caroline L Monteil, Marc Bardin, Cindy E Morris, Features of air masses associated with the deposition of Pseudomonas syringae and Botrytis cinerea by rain and snowfall, The ISME Journal, Volume 8, Issue 11, November 2014, Pages 2290–2304, https://doi.org/10.1038/ismej.2014.55
Murray, B. J., D. O'sullivan, J. D. Atkinson, and M. E. Webb. "Ice nucleation by particles immersed in supercooled cloud droplets." Chemical Society Reviews 41, no. 19 (2012): 6519-6554.
Pratt, Kerri A., Paul J. DeMott, Jeffrey R. French, Zhien Wang, Douglas L. Westphal, Andrew J. Heymsfield, Cynthia H. Twohy, Anthony J. Prenni, and Kimberly A. Prather. "In situ detection of biological particles in cloud ice-crystals." Nature Geoscience 2, no. 6 (2009): 398-401
Pratt, Kerri A., Paul J. DeMott, Jeffrey R. French, Zhien Wang, Douglas L. Westphal, Andrew J. Heymsfield, Cynthia H. Twohy, Anthony J. Prenni, and Kimberly A. Prather. "In situ detection of biological particles in cloud ice-crystals." Nature Geoscience 2, no. 6 (2009): 398-401
Prenni A J, Petters M D, Kreidenweis S M, Heald C L, Martin S T Artaxo P, Garland R M, Wollny A G and P¨oschl U 2009 Nat.Geosci. 2 402–5
Spracklen, D. V. and Heald, C. L.: The contribution of fungal spores and bacteria to regional and global aerosol number and ice nucleation immersion freezing rates, Atmos. Chem. Phys., 14, 9051–9059, https://doi.org/10.5194/acp-14-9051-2014, 2014.
Weinbauer, Markus G., Benjamin Guinot, Christophe Migon, Francesca Malfatti, and Xavier Mari. "Skyfall—neglected roles of volcano ash and black carbon rich aerosols for microbial plankton in the ocean." Journal of Plankton Research 39, no. 2 (2017): 187-198






Love this! I can envision planting tall trees along our waterways into the interiors while having 'rock beaver' parties doing small retention basins every 20 ft all the way back down from the hills.